How Flame-Retardant Nylon Achieves Self-Extinguishing: Mechanisms and Flame Retardant Principles
Aug 21, 2025
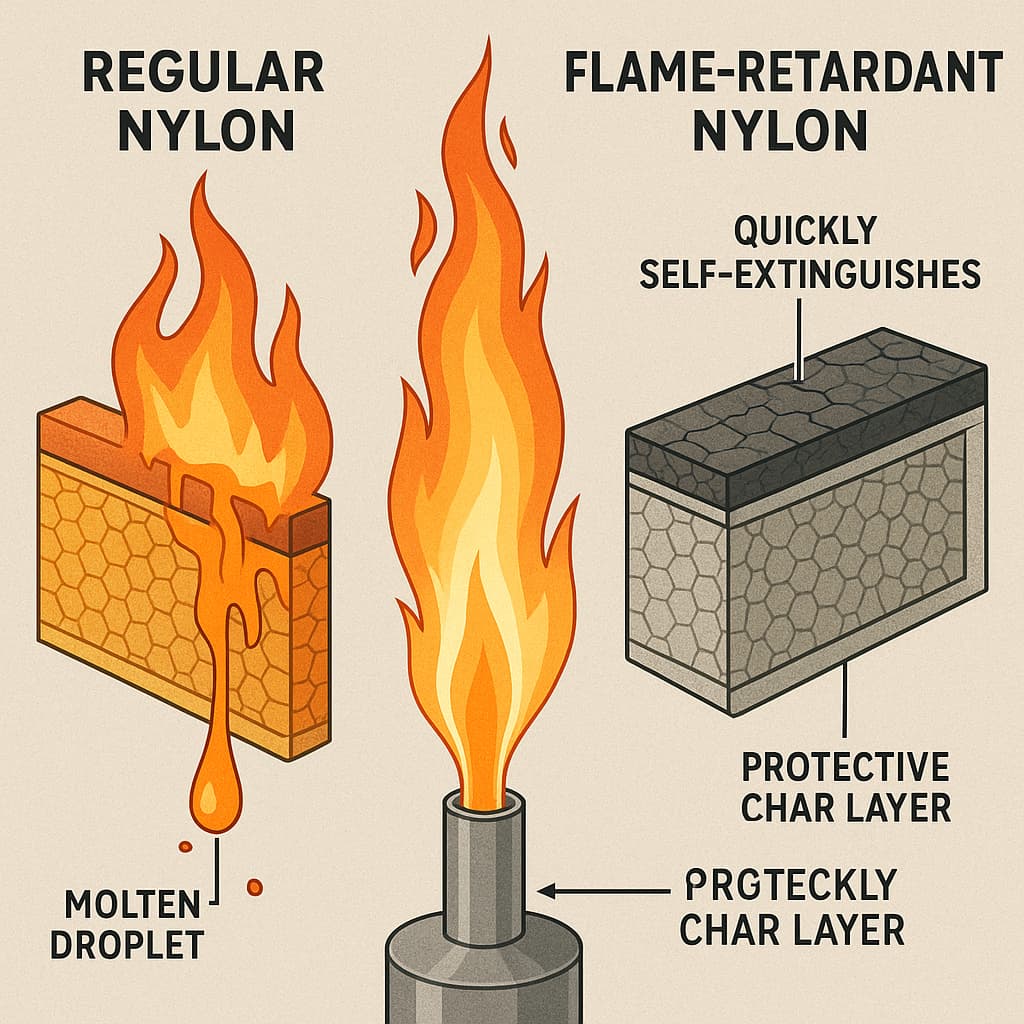
Nylon as a representative engineering plastic, is widely used in automotive components, electrical devices, and construction materials. However, due to its hydrocarbon backbone and amide groups, nylon is inherently flammable. Once ignited, it burns rapidly and may produce molten drips. For applications demanding high fire safety—such as electrical connectors, appliance housings, and automotive under-hood parts—pure nylon alone is insufficient. Flame-retardant nylon capable of self-extinguishing once the flame source is removed, provides a critical solution. But how is this self-extinguishing property achieved?
The fundamental mechanism lies in disrupting the chain reactions of combustion. Burning is essentially a process involving heat, free radicals, and oxygen. When the polymer decomposes, flammable volatiles react with oxygen to sustain the flame. Flame retardants act by interfering with this cycle. Some absorb heat, lowering the temperature; others release inert gases to dilute oxygen concentration; still others form a char layer that shields the polymer from oxygen and heat.
In nylon, the main flame retardant systems include halogenated, phosphorus-based, nitrogen-based, and inorganic fillers. Halogenated retardants, such as brominated and chlorinated compounds, release hydrogen halides during combustion, scavenging free radicals and terminating the chain reaction. Although effective, their toxicity and environmental concerns have led to restrictions in many industries.
Phosphorus-based flame retardants are now widely adopted. Upon decomposition, they produce phosphoric or polyphosphoric acids that promote char formation on the surface. The char layer blocks oxygen and heat transfer while reducing volatile release. Some phosphorus retardants also act in the gas phase, capturing free radicals for a dual effect.
Nitrogen-based retardants, such as melamine and its derivatives, work by releasing inert gases like nitrogen or ammonia during combustion. This dilutes oxygen in the flame zone and slows burning. Phosphorus-nitrogen synergistic systems are particularly effective, delivering strong flame retardancy at relatively low loading levels.
Inorganic flame retardants such as aluminum hydroxide and magnesium hydroxide decompose endothermically at high temperatures, releasing water vapor to cool and dilute the system. Though they require high loading, they are non-toxic and environmentally friendly, making them suitable for green flame-retardant nylon.
In practice, engineers often use tailored combinations. For electrical insulation, low-smoke halogen-free systems are preferred, typically phosphorus-nitrogen blends. In automotive components, balancing flame resistance with mechanical strength often requires glass fiber reinforcement with phosphorus-based retardants.
The self-extinguishing performance of flame-retardant nylon is commonly evaluated through standard tests such as UL94. Depending on whether the sample extinguishes quickly and avoids igniting cotton with dripping, materials are rated from HB to V-2, V-1, or the highest rating, V-0. These classifications are essential for product acceptance in safety-critical applications.
Looking ahead, stricter environmental regulations are driving halogen-free and low-smoke flame-retardant systems. Advanced phosphorus-nitrogen synergistic formulations, nano-scale retardants, and self-charring additives are emerging as next-generation solutions. They not only enhance safety but also expand nylon’s role in electric vehicles, 5G communication devices, and smart home applications.
Thus, flame-retardant nylon’s ability to self-extinguish arises from the combined physical and chemical effects of flame retardants. Understanding these mechanisms allows engineers to optimize formulations that balance flame retardancy, mechanical strength, and environmental performance, ensuring nylon’s continued relevance in safety-critical fields.
Read More