Selection Strategy of High CTI Nylon Materials in Electronic and Electrical Appliances
Sep 19, 2025
In the field of electronics and electrical appliances, high-CTI (Comparative Tracking Index) nylon materials are increasingly favoured by design engineers and materials scientists due to their excellent electrical corrosion resistance and insulation performance. Choosing the proper high-CTI nylon affects not only product safety, but also service life, reliability, and cost. Therefore, the selection strategy must consider multiple aspects comprehensively.
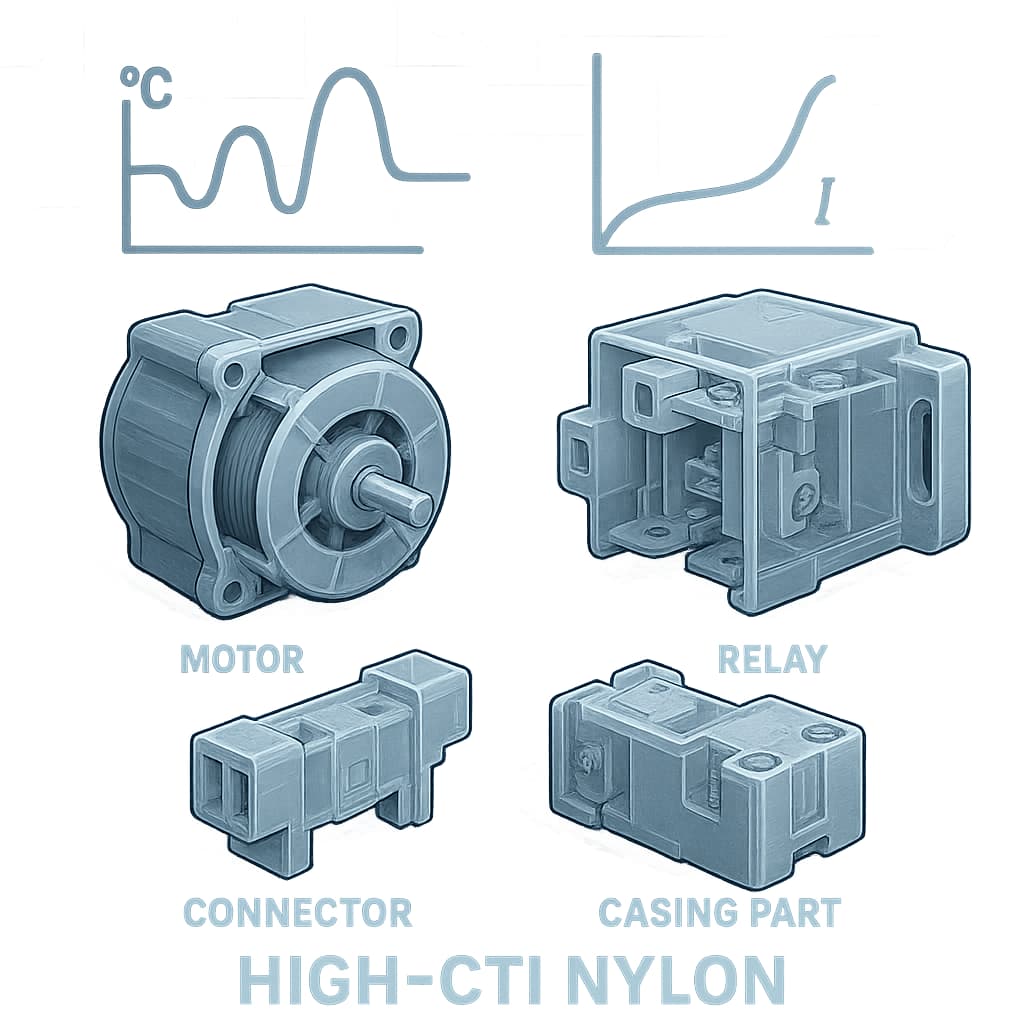
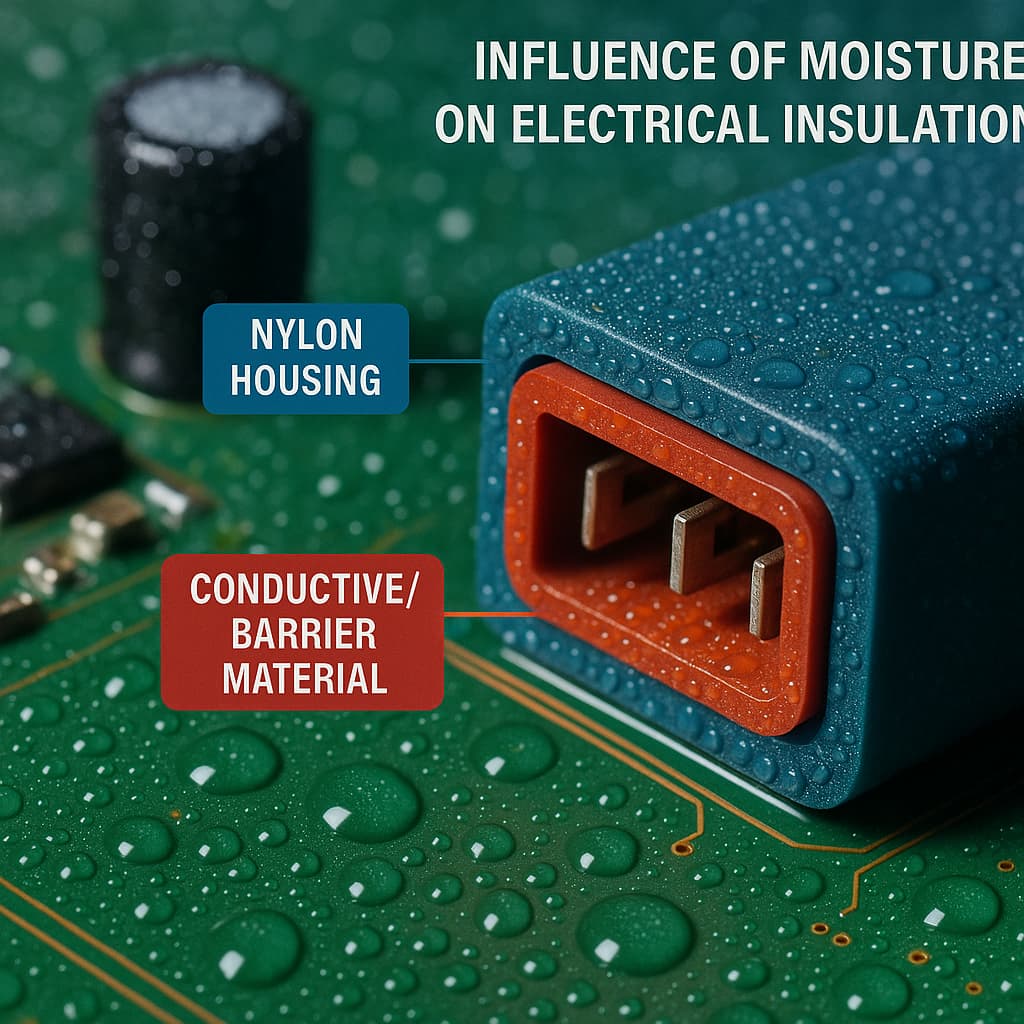
It is crucial to understand the physical meaning of the CTI metric. The CTI value reflects a material’s ability to resist surface tracking or electrical discharge under conditions of high humidity and pollution. The higher the CTI, the less likely a material is to develop arcs or conductive paths on its surface when exposed to damp conditions. This is especially important for housings, switches, sockets, and other components exposed to air that may contain dirt or moisture. Generally, a CTI value of 400 V or above is considered high-grade, suitable for outdoor or high humidity environments; for indoor consumer electronics, CTI values between 175 V and 250 V are common and often sufficient.
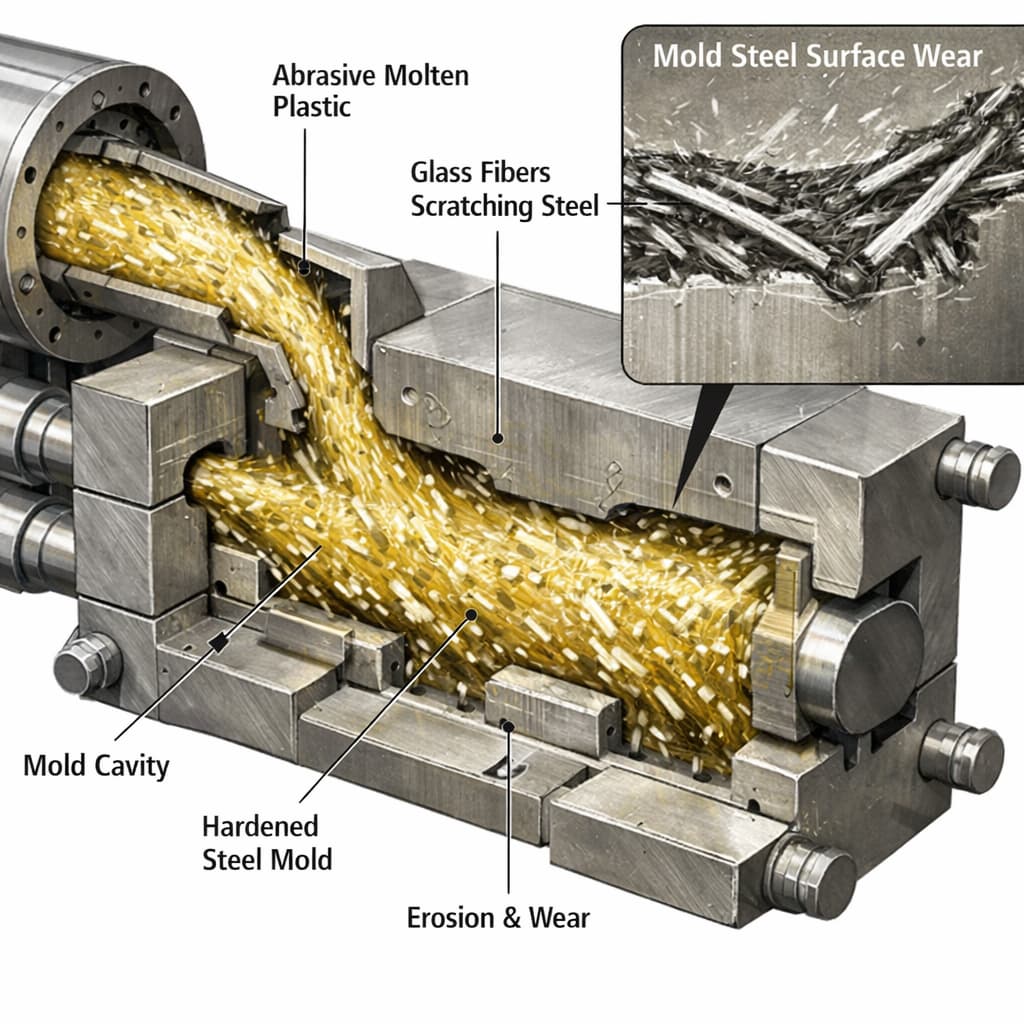
One must consider the material’s thermal performance and glass transition temperature (Tg). In electronics, the heating of circuit boards, components, and even the outer casing impose high temperature loads on materials. Although nylon (polyamide) naturally provides good heat resistance, its specifications vary greatly. You must examine both the continuous operating temperature and the transient peak temperature, and whether the CTI value degrades under high-temperature conditions. Also important is whether the material is modified with heat stabilizers or glass fibre reinforcements; these can enhance thermal performance, but may also affect electrical insulation (e.g. exposed fibres might alter surface corona propagation paths).
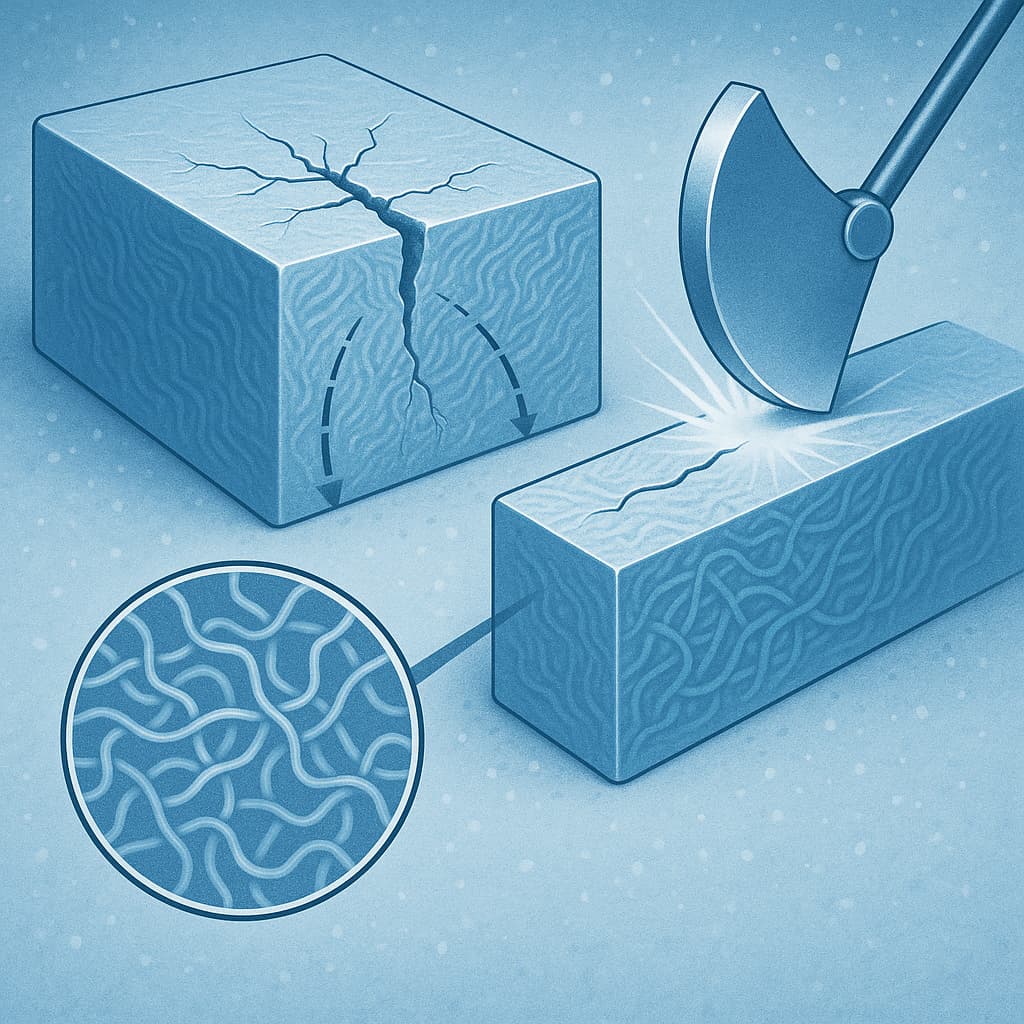
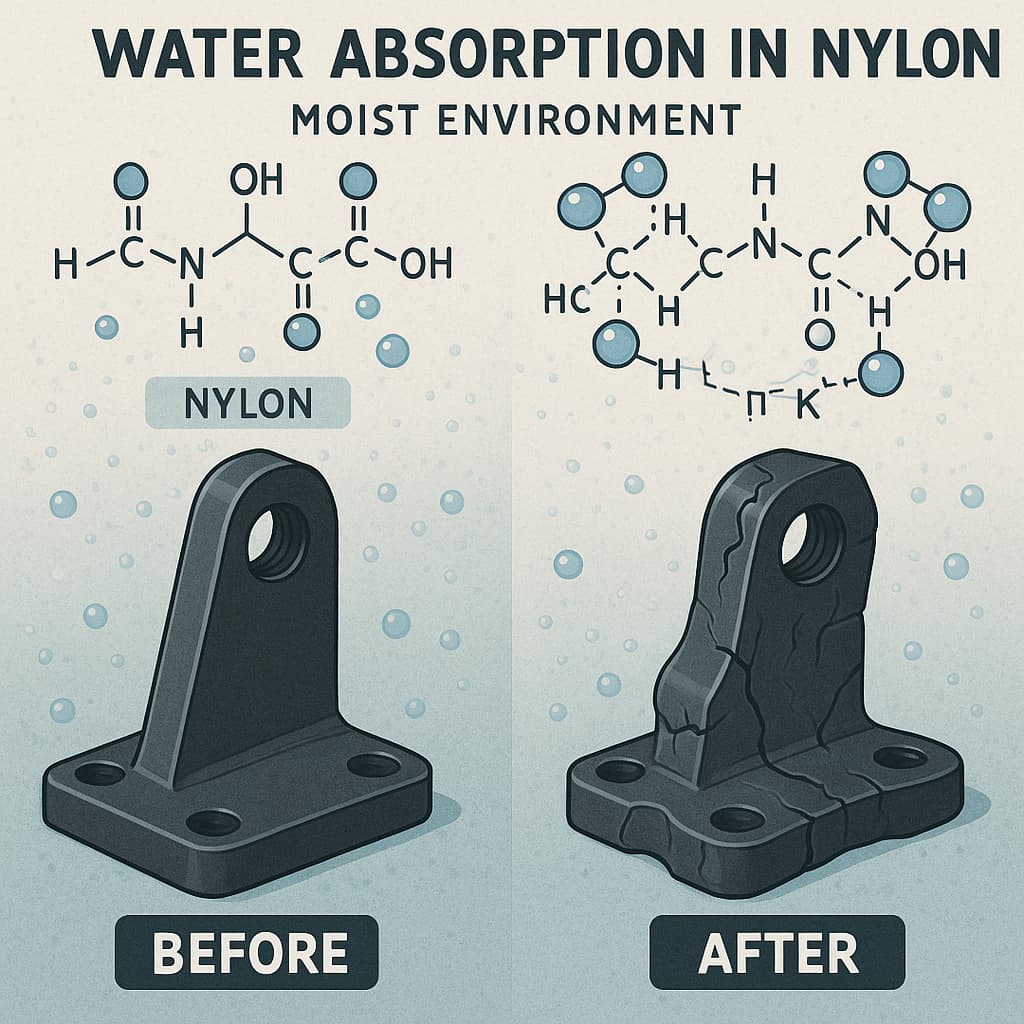
The moisture absorption rate and its effect on electrical characteristics cannot be ignored. Nylon tends to absorb water; when hydrated, its insulating properties deteriorate, volume swells, mechanical strength drops, and the CTI value may fall significantly. In practice, inspect how the material behaves under saturated absorption: whether its tracking or arcing resistance in soaked state remains acceptable. If the environment involves high humidity or rapid temperature changes, also consider performance after repeated wet-dry cycles. Some high-CTI nylons are modified (with carbon black or other additives) to reduce water uptake; although more costly, these materials are often more reliable under harsh conditions.
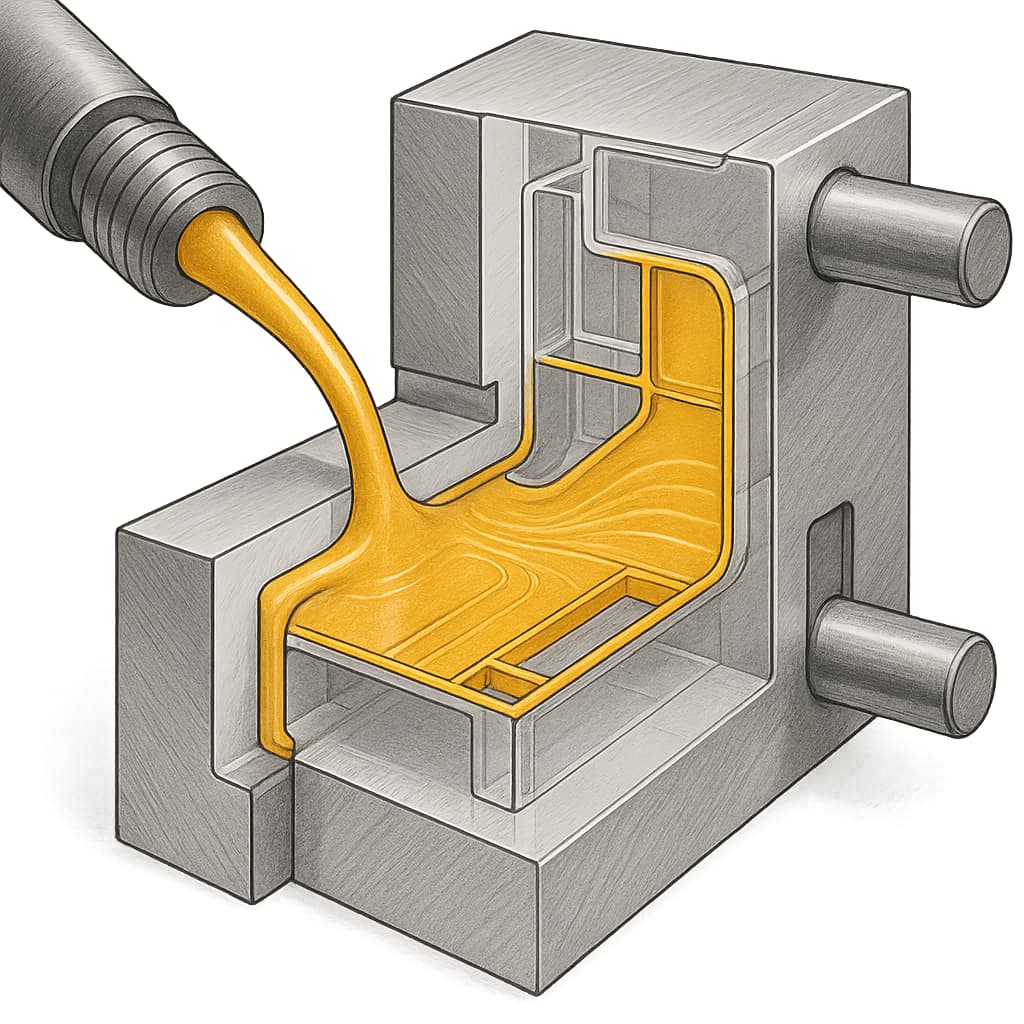
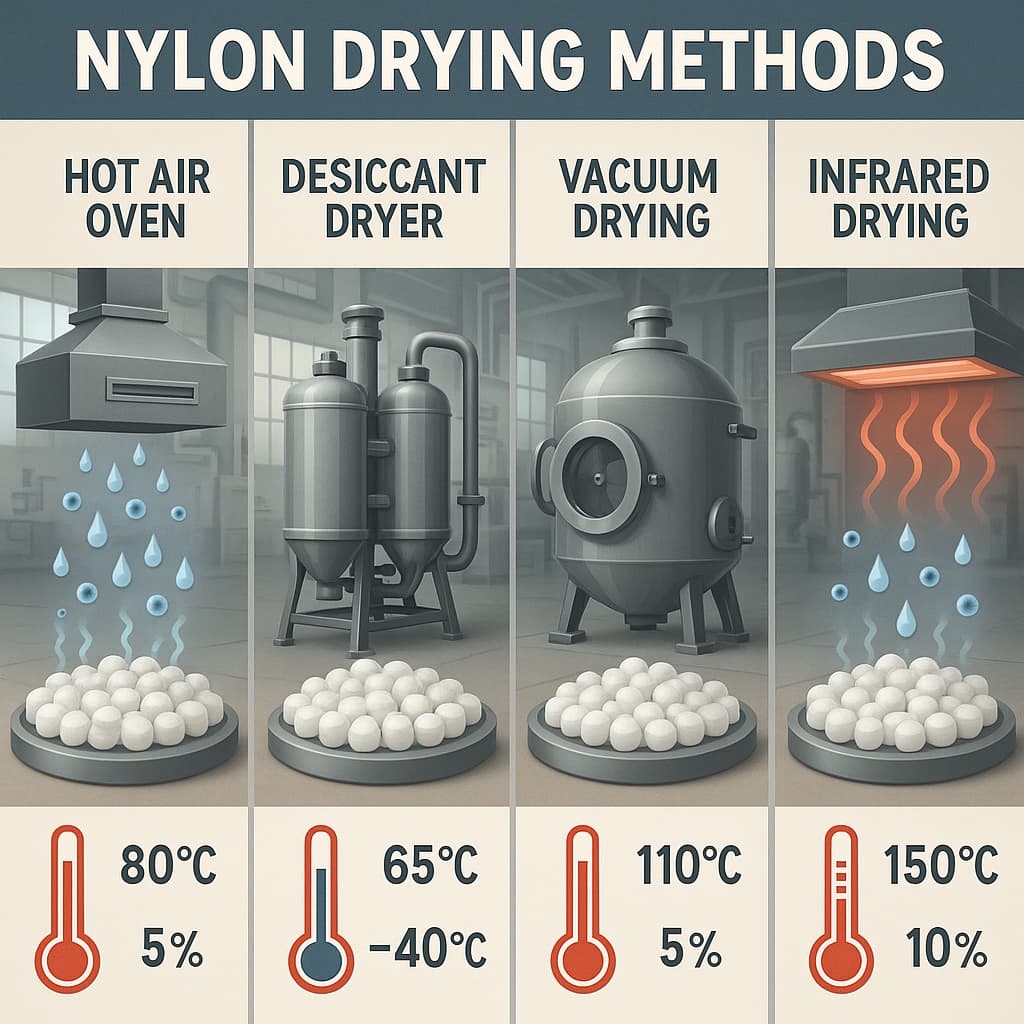
Processing behaviour and forming method requirements are important. Housings, pin seats, connectors, etc., are usually manufactured by injection moulding, extrusion, or other plastic forming processes. High-CTI nylon, particularly when filled (glass fibre, inorganic powders, carbon black) or weather-stabilised, may change melt flow behaviour, viscosity, melt flow index (MFI), and the melt temperature. These will affect mould design, wall thickness uniformity, demoulding difficulty, and surface finish quality. Poor flow may lead to short shots, weld lines, air bubbles, or sink marks. Thus, when selecting material, one must obtain from datasheets the melt index, melting temperature, processing temperature range, and ensure they match the equipment’s capability.
Long-term reliability and environmental regulation must be considered. Products in this sector often require lifetimes of several years or more. The performance degradation over time under temperature, humidity, and electrical stress is expected. Key issues are whether high-CTI nylon will oxidise, yellow, embrittle, or crack. Also it must comply with regulations such as RoHS, REACH: using non-toxic flame retardants, not containing prohibited substances; additives should not compromise recyclability. Also one should check whether the supplier provides accelerated ageing test data (high temperature, high humidity, voltage cycling) and whether the material sample is certified under UL or IEC standards.
Cost and supply chain stability should not be underestimated. High performance nylon often carries higher costs for raw materials, fillers, colorants, safety flame retardants than standard nylon. Design teams must balance performance requirements with cost budget. In mass-produced equipment like household appliances, power adapters, communication devices, material cost and processing efficiency directly influence the overall cost. Also, supplier lead time, batch-to-batch consistency (variation in performance between lots) can directly affect manufacturing reliability. Choosing a reputable high-CTI nylon brand, understanding its global or local inventory, and having alternative sources to cover supply disruptions are hallmarks of mature material selection strategy.
Comprehensive testing and prototype validation are indispensable. Theoretical datasheets are instructive, but actual performance in end-use is influenced by environmental conditions, structural design, wall thickness distribution, surface finish and more. Design engineers should request material samples and conduct real assembly testing in expected environments, including extreme temperature/humidity cycling, dielectric withstand tests, surface tracking tests, thermal shock, mechanical strength tests, etc., to verify the material’s behaviour in specific applications. Also allow design margin to accommodate performance degradation.
In summary, selecting high-CTI nylon materials in electronics and electrical appliances is a multi-factor trade-off: one must look beyond just insulation metrics to consider thermal resistance, moisture absorption, processability, reliability and regulatory compliance. Only when performance, cost, manufacture, and regulation are all balanced can the final product achieve safety, longevity, and market competitiveness.
Read More