Application Prospects of AI and Digital Twin in Nylon Modification R&D
Nov 26, 2025
The integration of advanced computing technologies with material science is reshaping the landscape of nylon modification. Historically, development in this sector relied heavily on experience-based trial-and-error, long experimentation cycles, and incremental formula iteration. The emergence of artificial intelligence and digital-twin technology is pushing the industry toward a data-driven research model that offers greater accuracy, shorter development time, and significantly lower costs. Nylon modification, with its complex interplay of raw materials, additives, processing parameters, and performance targets, is particularly suited to this transformation.
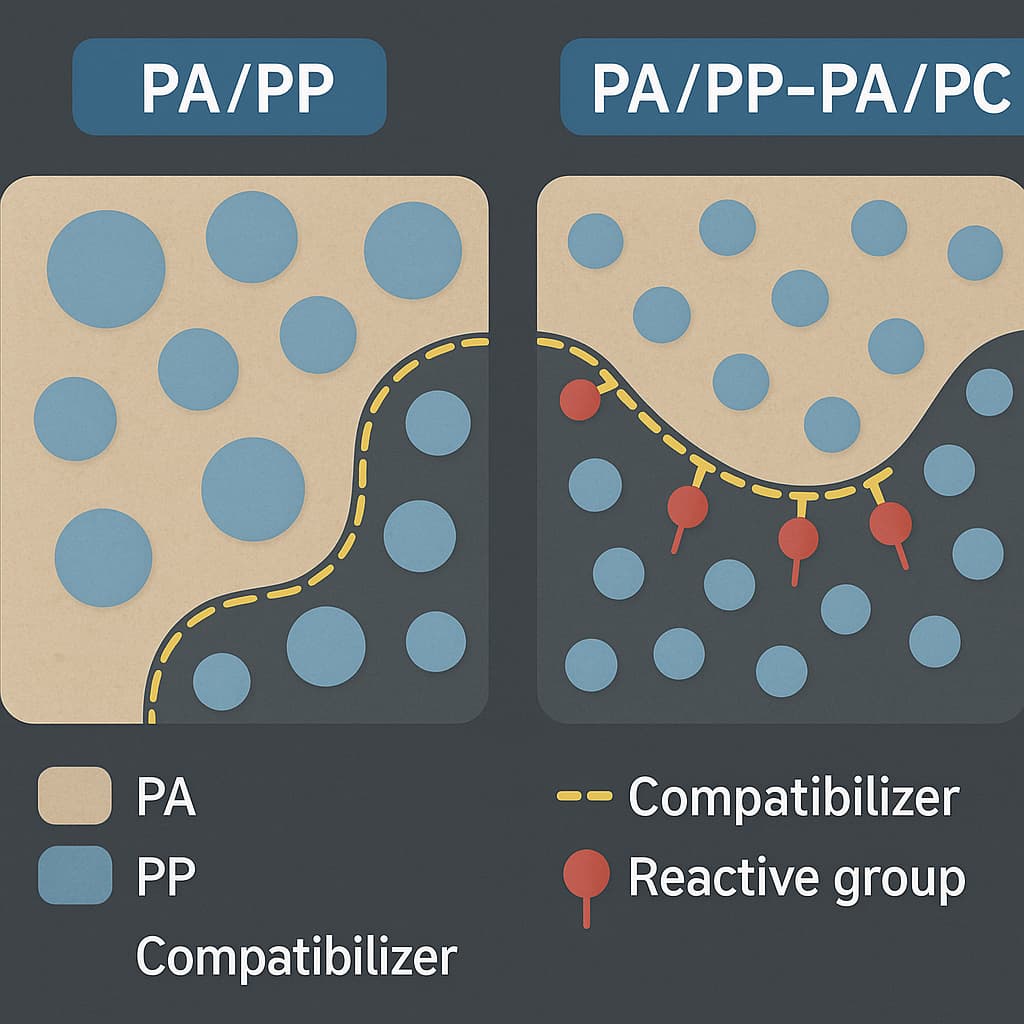
AI algorithms allow researchers to establish structure–property correlation models based on historical experimental data, processing parameters, and performance results. Through feature extraction and nonlinear fitting methods, AI can identify the key factors influencing material behavior, such as the interaction between glass-fiber content and interfacial compatibility, the influence of impact-modifier systems on crystallization kinetics, or the competitive effects between flame-retardant additives and stabilizers. While human engineers often find it difficult to analyze multiple interacting variables simultaneously, machine-learning models can evaluate thousands of potential combinations within seconds and recommend the top candidates that meet mechanical, thermal, rheological, or flame-retardant requirements. This capability significantly reduces redundant experiments and accelerates development cycles.
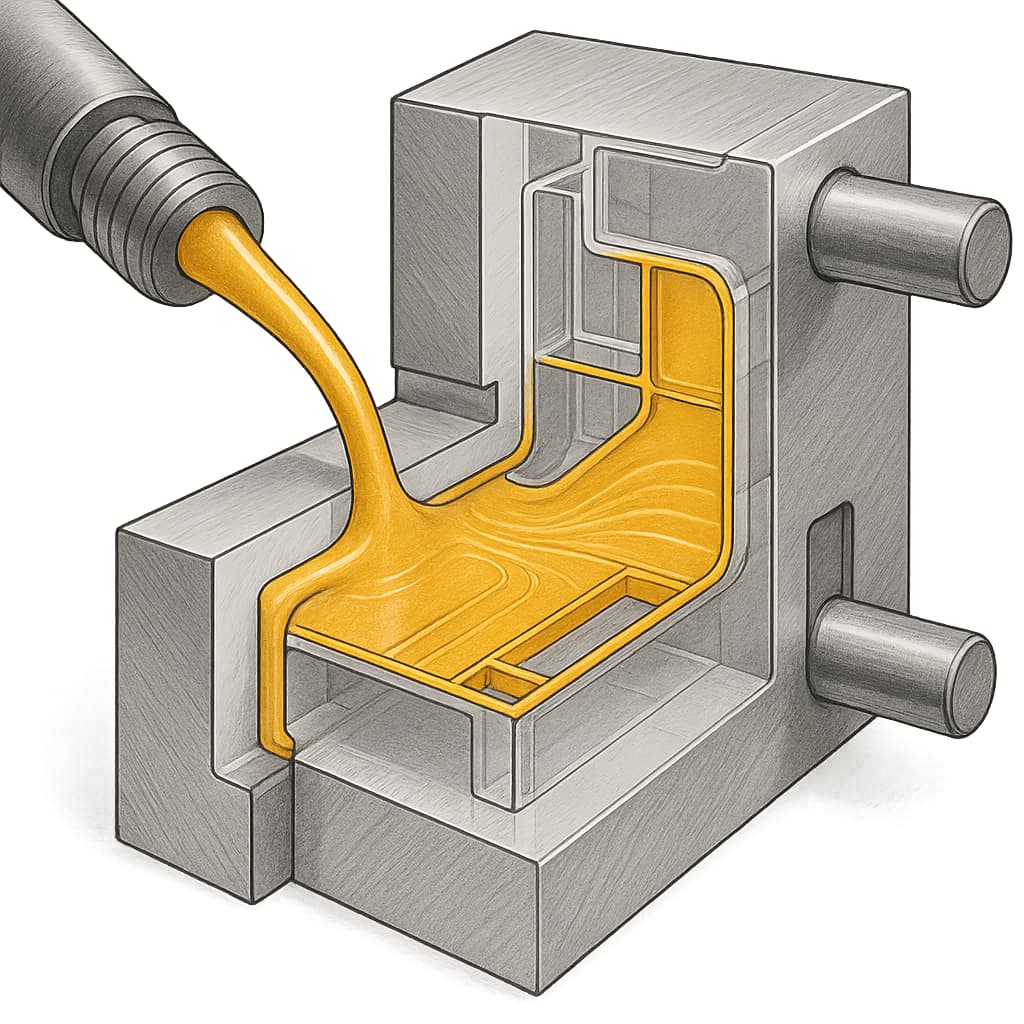
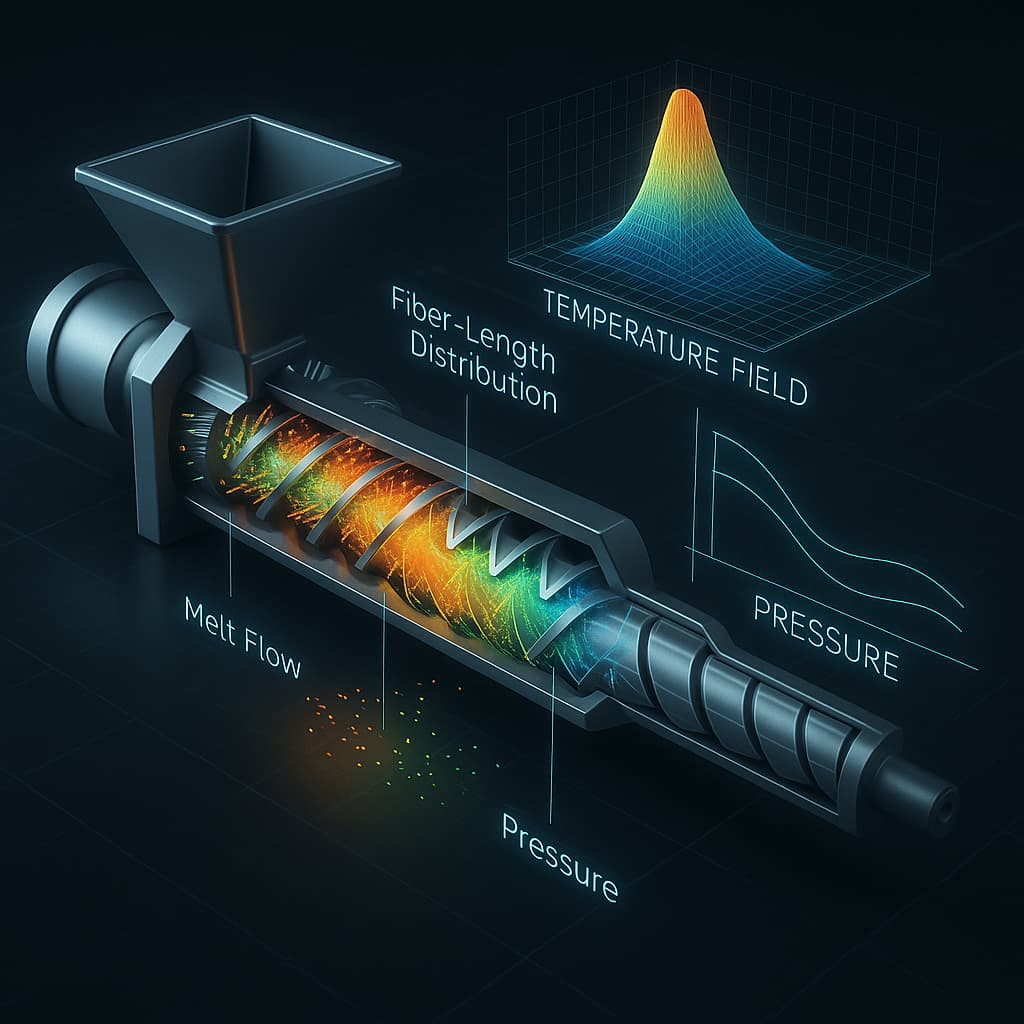
Digital-twin technology deepens the virtual-engineering framework by creating dynamic models that replicate the structure and behavior of actual equipment. In nylon compounding, digital twins can simulate extrusion processes, including glass-fiber breakage ratios, fiber-length distribution, melt-temperature gradients, shear-rate distribution, and pressure fluctuations along the screw. Such insights allow engineers to optimize screw profiles, maximize fiber retention, and reduce energy consumption. In injection-molding applications, digital twins can accurately predict melt-front progression, cooling dynamics, shrinkage behavior, and warpage tendencies—capabilities especially valuable for highly filled nylon grades or complex flame-retardant systems. Compared with traditional CAE simulation, digital twins emphasize bidirectional coupling, enabling real-time calibration based on actual machine data.
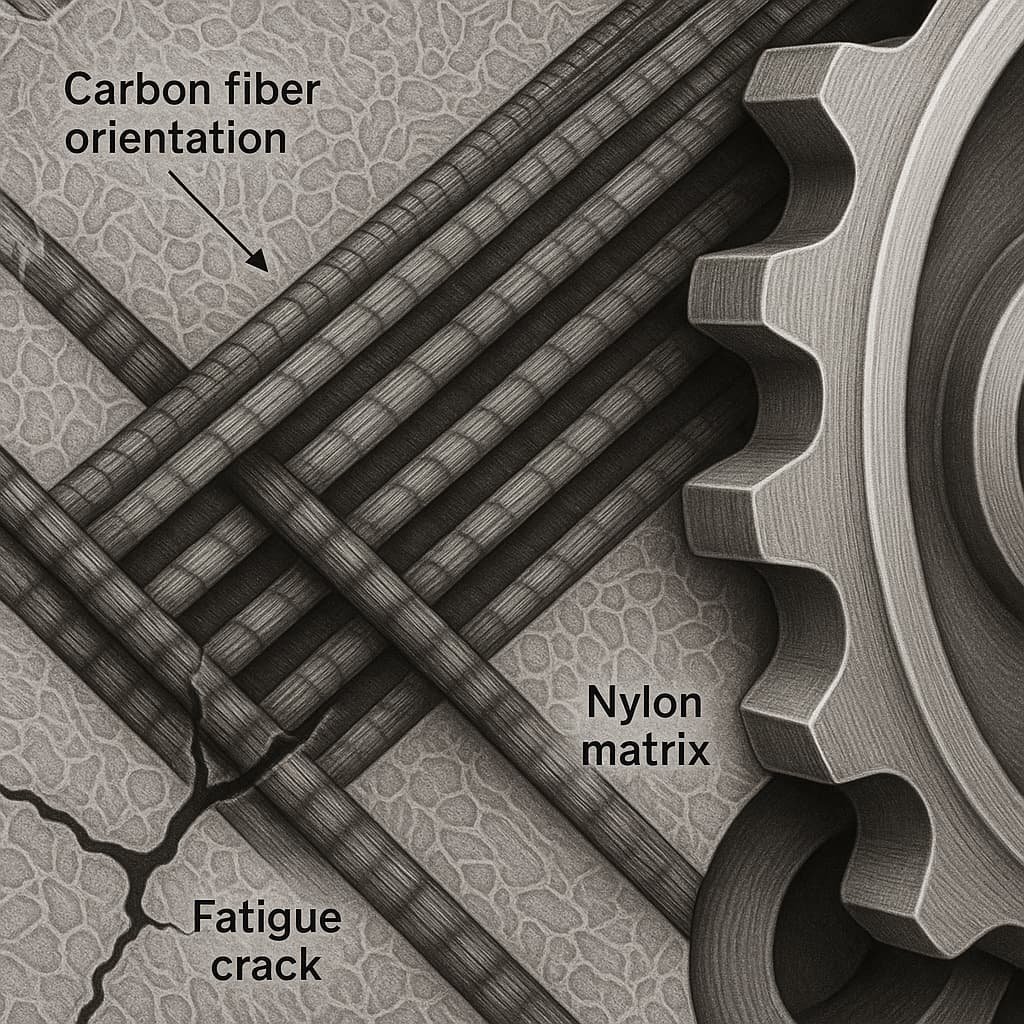
As data accumulation grows, AI becomes the core of a closed-loop R&D ecosystem. Processing data, mechanical testing results, thermal analysis parameters, microscopy observations, and long-term aging performance can be continuously integrated and used to refine predictive models. For composite formulations such as PA66 GF50, PA6 carbon-fiber composites, or PA6/PA66 blends, AI can detect subtle microstructural variations—including changes in crystallinity, fiber-matrix adhesion, internal stress distribution, and melt-flow anomalies. When combined with digital twins, AI can recommend optimal processing windows, such as melt temperature, screw speed, back pressure, residence time, or drying conditions, ensuring stable mass-production quality.
The value of AI-assisted material development becomes even more significant when addressing customized performance requirements. Customers increasingly demand fine-tuned materials for specific applications: high strength and heat resistance for structural automotive parts, flame retardancy with minimal warpage for electronic components, or wear resistance with dimensional stability for industrial gears. AI multi-objective optimization can identify the most feasible formulations among thousands of possibilities, while digital twins validate these solutions under realistic manufacturing conditions. Furthermore, AI can analyze failure cases provided by customers—such as insufficient flow, fatigue cracking, mechanical degradation, dimensional instability, or excessive warpage—and propose data-supported improvement strategies.
Looking ahead, nylon modification is expected to transition toward a highly interconnected and intelligent R&D ecosystem. Data from production equipment, testing laboratories, and supply chains will converge into unified material-informatics platforms. AI models will automatically adjust formulations according to process conditions, equipment configurations, and regional industry requirements. Full digital-twin factories will enable engineers to simulate entire production lines—from drying to compounding, from molding to final inspection—ensuring that every step is optimized before real-world production begins. As modeling and algorithmic precision continue to improve, this digital transformation will become central to enhancing competitiveness, reducing costs, and accelerating innovation.
In conclusion, AI and digital twins represent a transformative force within nylon modification. They shift the development paradigm from empirical trial-and-error toward predictive, data-centric engineering. As more companies build data infrastructures, implement advanced monitoring systems, and integrate software with processing equipment, these technologies will rapidly become standard practice and shape the next evolution of material research and industrial manufacturing.
Read More